Some thoughts on the FDA's briefing document for the Novavax EUA application
Will the FDA make Novavax a scapegoat for the sins of Moderna and Pfizer?
[Updated June 7, 2022. I’ve re-written the section on the cross-over period. My hunch was correct — it essentially wipes out the control group after 49 days. But they did *not* cross over to Moderna and Pfizer, they crossed over to the opposite of whatever they had in the pre-crossover period — placebo or Novavax. It’s a very strange study design that raises massive red flags.]
On Friday, the FDA released its Briefing Document in connection with the emergency use authorization application for Novavax. Everything about the Novavax clinical trial and briefing document is weird.
Novavax bills itself as a “Recombinant Spike Protein Nanoparticle Vaccine with Matrix-M1 Adjuvant” and just hearing those words I’m like Justin Bieber on TikTok,
Immediately no, immediately no, immediately no, I’m telling you right now, I’ve seen what I needed to see... immediately no.
Carolyn Johnson, a reliable stenographer for the cartel at The Washington Post gushed about Novavax in an article that read more like an advertisement. She called it “a classic decades-old technology,” “an older and more familiar technology,” and then this doozy: “Protein-based shots are tried and true. They are used against influenza, hepatitis B, [HPV], and shingles.”
This is all complete nonsense. According to the CDC’s own data, the influenza vaccine does not work. The hepatitis B vaccine is a major factor in the explosion of autism cases since it was introduced in the 1980s. HPV was the most toxic vaccine on the market prior to the introduction of Covid-19 shots. And the demand for shingles vaccine was created by the varicella vaccine that is non-sterilizing and thus prevents society from developing herd immunity against varicella zoster virus.
The spike protein in Novavax is grown in cells from the fall armyworm moth. WaPo even produced an instructional video on How Novavax uses moth cells to create its coronavirus vaccine.
What could possibly go wrong? Yes I looked it up and it turns out that there are indeed insect retroviruses. In 20 years will we see scientists announce, just like they did with polio vaccines, ‘oh darn we didn’t realize we were injecting dangerous retroviruses into millions of Americans with this miracle new vaccine!’?
No one knows how Matrix-M works. In general, adjuvants are bad news because they overstimulate the immune system and they have been linked with all sorts of chronic illnesses including life threatening allergies and autoimmune disorders.
So already out of the gate I’ve got concerns.
The trial started out well enough — a decent sample size — 29,582. Not as big as the polio field trial (1,800,000 participants) but certainly better than the preposterous junk science recent Pfizer booster trial in kids 5 to 11 (that looked at blood samples from just 30 participants).
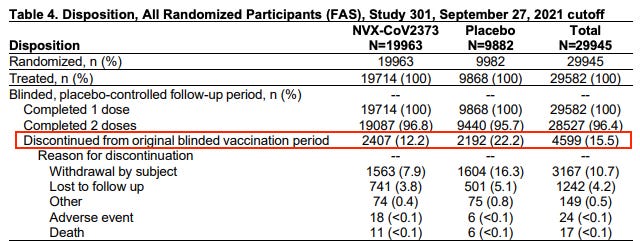
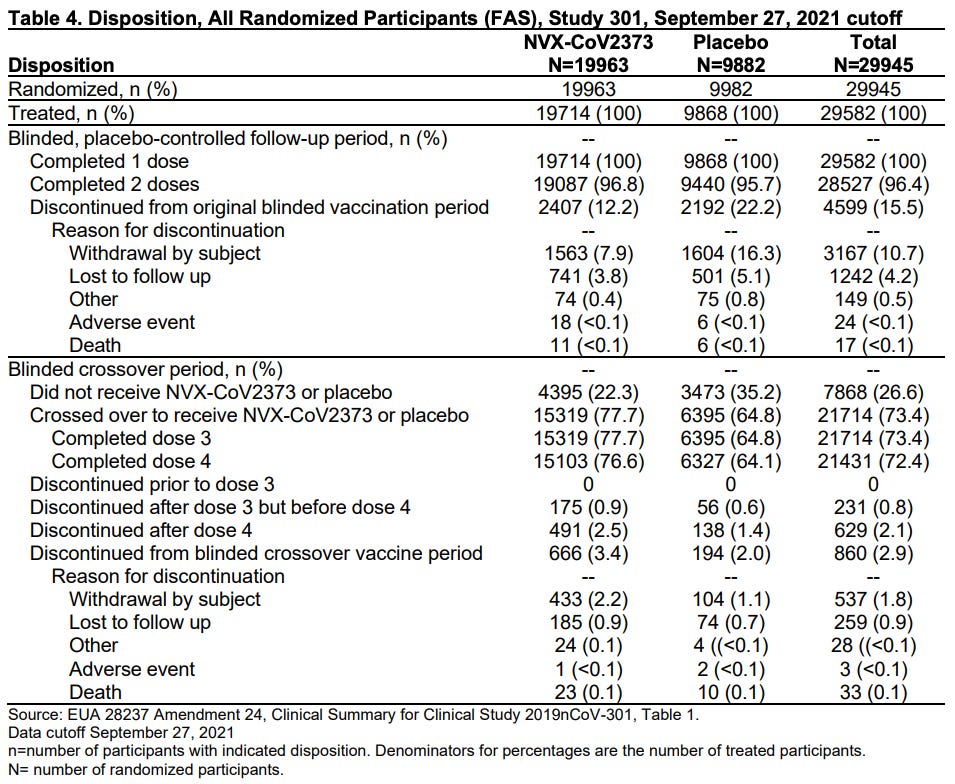
Things went south very quickly for Novavax. They lost a stunningly large percentage of participants. If I am reading Table 4 correctly, they lost 12.2% of treatment group participants and 22.2% of placebo participants. This should not happen and, if this is correct, it is instantly disqualifying.
Novavax claims that the placebo was saline. But the fact that 22.2% of placebo group participants quit suggests something was seriously amiss.
Making matters worse, at 4 of the 5 trial sites, the vaccines used in the clinical trial were manufactured by Emergent BioSolutions. As you may recall from recent headlines, Congressional investigators discovered that Emergent BioSolutions produced 400 million contaminated coronavirus vaccine doses. At one point last year federal regulators seized the Maryland plant and handed it over to Johnson & Johnson. And now even J&J has walked away.
Emergent BioSolutions even bought a member of the CDC’s Advisory Committee on Immunization Practices, Wilbur Chen, for $418,428, but it was not enough to save them.
In the briefing document, the FDA assures us that the data from the Novavax clinical trials that used doses manufactured by Emergent BioSolutions were excluded from their analysis (see discussion, p. 10). But there were 4 sites (U.K., South Africa, Australia, and Australia/U.S.) that used doses manufactured by Emergent BioSolutions and the FDA only mentions excluding 3 sites from the analysis. So I’m not buying the FDA’s story here.
Then the trial did something bizarre — “blinded crossover.” Reading the briefing document it feels like Novavax intentionally messed up their own trial. The question is why? Here’s how the FDA describes it:
During the course of the study, COVID-19 vaccines authorized for emergency use became available, and participants (when eligible for vaccination per national and local public health prioritization recommendations) were offered the opportunity to cross over from the originally assigned study treatment to the other study treatment (vaccine or placebo) in a blinded fashion… (p. 5).
In plain English… during the Novavax trial, Pfizer and Moderna coronavirus shots received emergency use authorization from the FDA. So Novavax allowed their own trial participants to switch from whatever group they were currently in (placebo or treatment) to the opposite group (placebo switched to treatment and treatment switched to placebo). But because it was a blinded crossover — the participants did not know which group they were in (either before or after crossover).
This maneuver renders the Novavax data almost unreadable. Instead of 4 categories of data (treatment group dose 1, treatment group dose 2, placebo group dose 1, placebo group dose 2) there are now 8 categories of data (treatment group dose 1, treatment group dose 2, placebo group dose 1, placebo group dose 2, crossover treatment group dose 3, crossover treatment group dose 4, crossover placebo group dose 3, crossover placebo group dose 4).
But remember, Novavax is losing participants throughout these trials as people quit and walk away. So the number who start each step is not the number that complete each step. This is what that looks like and I confess I’ve stared at this chart for a long time and it still does not make sense (if you understand it, please explain what’s going on here in the comments).
It also seems like a crazy number of people died in this trial. 34 across all of the treatment groups and 16 across all of the placebo groups. Am I reading that correctly?
It seems that the effect of the blinded cross-over is that the RCT only existed for 49 days. The cross-over wiped out the control group, so it’s impossible to track long term harms. The moment someone crossed over they harvested the data on that day and washed their hands of the person. From the FDA briefing document:
The primary efficacy endpoint was assessed until a participant received the first blinded, crossover vaccination or until the data cutoff of September 27, 2021, whichever came first.
If you have other theories of the case please let me know in the comments.
All of the articles in the mainstream media have focused on the fact that Novavax appears to triple one’s risk of myocarditis (6 cases in the treatment group, 1 in the placebo — but the placebo group was half the size of the treatment group). The FDA briefing document (p. 6) states:
Multiple events of myocarditis/ pericarditis were reported in temporal relationship to NVX-CoV2373 [Novavax] administration, similar to myocarditis following mRNA COVID-19 vaccines and raising concern for a causal relationship to NVX-CoV2373.
Novavax has already put out a statement saying it wasn’t the vaccine’s fault, ‘there’s just a lot of myocarditis going around these days, blame a virus.’ Overall there were 76 cardiac events reported in the treatment group and 34 events reported in the placebo group (suggesting a smaller but still significant increased risk).
I just want to close with a thought about the politics of this decision (VRBPAC is meeting tomorrow at 8:30 a.m. Eastern to discuss this application).
The FDA clearly favors Moderna and Pfizer which suggests that something untoward is happening behind the scenes.
Fauci has been publicly pouring cold water on the Novavax vaccine for months and everyone knows that Fauci controls the money for federal research grants.
It will be very interesting to see what VRBPAC does with this incomprehensible application. On the one hand, they may subject Novavax to more serious scrutiny than Moderna and Pfizer and turn Novavax into a scapegoat. If Novavax is not authorized then the FDA can claim that they really do care about vaccine safety.
But Moderna and Pfizer Covid-19 shots are just as dangerous if not worse than Novavax. So if they subject Novavax to appropriate scrutiny it opens up the possibility that Moderna and Pfizer may eventually be subjected to similar examination.
My sense is that in general, the thought of a control group frightens the FDA — whether that control group is unvaccinated Americans or Americans vaccinated with a different type of Covid-19 vaccine — because control groups open up the possibility that the mRNA shots will be further revealed to be complete and total junk.
Lest anyone think that Novavax is the good guy in any of this… the Washington Post revealed that if Novavax is authorized in adults, the company “plans to seek expanded authorization for use of the shot in adolescents and as a booster.”
If the Future Framework is approved on June 28, next generation reformulated versions of Novavax will skip clinical trials in perpetuity.
Because Novavax is a protein subunit vaccine it is unlikely to be sterilizing which means that it will continue to drive the evolution of variants that evade vaccination.
(Updated: Filip Dubovsky, Executive Vice President, and Chief Medical Officer at Novavax claims that Novavax is sterilizing in primate studies. Based on the historical record of protein subunit vaccines I am deeply skeptical that these shots will be sterilizing in humans. I’d absolutely love to be wrong about this.)
The pandemic will never end as long as the FDA continues to rely solely on vaccines.
As always, the only way out of the pandemic is to pivot to therapeutics.
Blessings to the warriors. 🙌
Prayers for everyone fighting Pharma fascism around the world. 🙏
In the comments, please let me know your thoughts on the Novavax application.
As always please help me to correct any typos. Of course, unlike the FDA, I always welcome corrections to any factual points as well.






I wonder, at this point, exactly how many unvaccinated Americans are really merely holding out for a "better" or "more traditional" vaccine. Certainly I was hearing that earlier in the pandemic, but now? Pretty sure most of us have either lost our faith in the ability to vaccinate against Covid entirely (if we ever had it), lost our faith in the regulatory process (if we ever had it), or lost our fear of the virus itself (if we ever had it).
Easy for them, at this point, to put on a show surrounding Novavax and pretend to be grown-ups doing their jobs, either because they already won (most people are mRNA vaccinated!), or because they already lost (their vaccines are awful, public trust is eroded, and and now the CDC is reporting that millions of children "missed" their regular childhood vaccines during the pandemic).
Yes, therapeutics. It always should have been therapeutics Toby. I wish everything you write would make national news.